26/10/09:
-
8 WIK individuals (born 02/07/09) separated in two acuarium (4:4)
- A set of pre-juveniles (born 01/09/09)
26/10/09:
>> load(‘c:\hipertec\collectivebehavior\bayesiano\datos_wardetal.mat’)
I use the parameters found yesterday as optimal ones:
>> params=[0.5271 0.9003 0.4316 0.5311 ];
Now, I use the following simple rule: When the predator is present, the prior probability of predator is one. True, this will result in probability 1 for that side, no matter what other fishes do. But we may still have some fishes going towards the predator, due to the gaussian noise in the decision. Also, the probability of going towards the predator side increases the more fish go that way. One interesting (and probably unrealistic) prediction of this model is that the number of replicas avoiding the predator have no effect, because the probability for that side is already 1.
Results of the model are not terrible. Some are remarkably better than those of Ward et al. 2008 (Figure 4). In particular, from the four figures below, Fig. 1, Fig 2B, Fig 3A,B work better than Ward et al. Unfortunately, Fig. 2A and Fig. 4 work clearly worse.


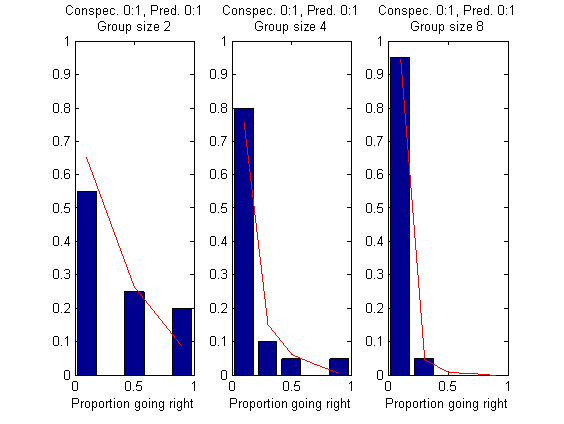

Note that we still have room for improvement: First, the model’s parameters were fit only for the case without predator. Second, Ward et al. fit the parameters separately for the cases with and without predator, so at this point they have twice as many parameters as us. In fact, they use an experiment to fit their parameter r:l reflect the presence of the predator, and we have not done such a thing (we just set the prior to 1).
Another possibility is to introduce the bias in the random number instead of in the prior. At this point I find this option less elegant, because I still do not see where exactly that random number might come from.
Between the 24 and 28 hours of life, it is needed to bleach the embrios, in order to avoid fungii. This is made as follows:


After this, the eggs are put in a new petri dish with E3 medium (as in Collection of zebrafish eggs) and stored in the 28ºC incubator. Before or after this procedure, you can remove the dead eggs. Dead eggs are visually different from the ones that are alive. By simple visual inspection, the dead eggs look whitish and the healthy ones look yellowish. Under the dissection microscope, dead ones have dirty nuclei while healthy ones have embryos moving inside.
Results obtained with the following hypotheses: A Bayesian estimator finds the probability that there is something interesting at one side, based on how many fishes have gone that way. This estimator has 3 parameters: Probabilities of true positive and true negative for the other fishes, and absolute probability that something interesting is at that side. We have two of these estimators, one for each side. Then, the fish decides to go to the side with highest probability of being interesting, but we add a random number from a normal distribution to one of the probabilities to introduce stochasticity. The variance of this random number is the fourth parameter of the model.
I fit the four parameters for best comparison with the results presented by Ward et al. (2008) Quorum decision-making facilitates… PNAS, in their Figure 2.
>> params=fitparametros_bayes(hist_wardetal,1000);
>> params
params =
0.5271 0.9003 0.4316 0.5311
I only run 10 iterations for the fitting. Also, I think that the lines in the graph above should not cross, and also should decrease monotonically. So the fit must be improved.
However, I would say the results are good. Bars are experimental data from Ward et al. 2008, lines are model predictions. Compare with Figure 2 of Ward et al. 2008:







All these results are without replica predator. I will introduce the predator tomorrow.
After fish have laid eggs, we have the aduls fish in the reproduction aquarium (the one with a net in the bottom, in Spanish “paridera”), and the eggs have fallen below, on the outer aquarium.
1- Remove the fish with a net, and put them in another aquarium.
2- Remove the reproduction aquarium. For washing, I think they just use tap water and they let it dry.
3- Pass the water of the aquarium through a strainer (“colador”) (vamos, que la colamos). The strainer is a normal small one, baught in a “chino”. The water is thrown away, the eggs remain in the strainer.

4- Fill a petri dish with aquarium water (this may be done with the same aquarium, just after the fish have been removed).
5- Reverse the strainer (forcing the net if it is a rigid one), and submerge it in the petri dish. In this way, the eggs detach from the strainer and go into the water.





Then, we go to the lab.
6- Fill another petri dish with E3 medium. This petri dish should be a new one.
7- Cut the tip of a plastic pipette, so that it gets a bit wider.
8- With the plastic pipette, and under a disection microscope, take the eggs from the petri dish with water and put them in the one with E3 medium. Take care not to take too much dirt from the first petri.
9- Store the eggs at 28ºC.
Taken from the book “Zebrafish”, edited by Christiane Nüsslein-Volhard and Ralf Dahm, page 22.
E3 medium for standard work with embryos:
1- 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, 10-5 % Methylene Blue.
2- E3 can be made up as a 60x stock (without Methylene Blue). The 1x medium keeps under non-sterile conditions at room temperature for over a week.
3- For 10 litres of a 60x stock solution, mix 172 g NaCl, 7.6 g KCl, 29 g CaCl2.2H2O and 49 g MgSO4.7H2O. Store stock in a fridge.
4- Dilute 160 ml of 60x stock in distilled H2O (dH2O) to make up 10 litres of E3 (1x), and add 30 ml of 0.01% Methylene Blue as a fungicide.
We have filled our 50 l aquarium with treated water of the Cajal Institute Animalario and sand from a pet store. It is important to clean the sand before setting the aquarium, otherwise the water becomes very dirty.
We followed the manuals for the water system and thermostat, and set the temperature to 28ºC.
